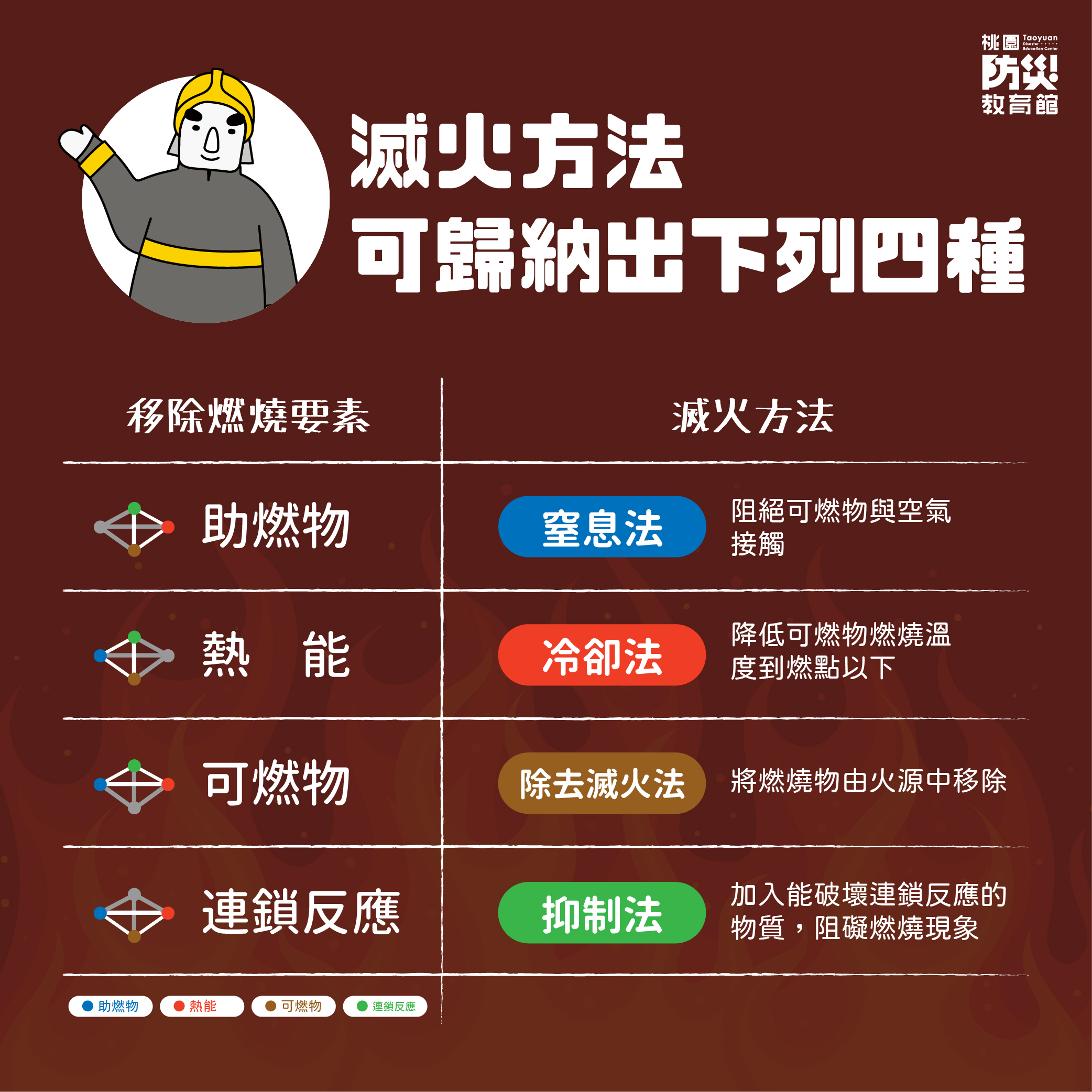
Fire Basic Science:Four Components
The chemical reaction maintaining aburning fire requires four components, which are:
A comburant(oxygenorair), heat (temperature), combustible material, anda chain reaction. Remove any one component and combustion cannot occur.
Therefore,the basic principle for putting out a fire is to remove one of the componentsof the chemical reaction, so that the fire cannot be sustained. The followingfour methods can be used to put out a fire:
1.Smothering: Separate the combustible material and the comburant(air).This can be done by:
(1) Smothering with incombustiblegas: spray incombustible gas (carbon dioxide or nitrogen (CO2 or N2)) into the burning material toseparate the burning material from the oxygen.
(2) Incombustible foam: when thefoam comes in contact with the burning material, the foam will release steam(from evaporated moisture) while the foam itself will block off air supply.This can achieve effective separation.
(3) Covering with incombustiblesolids: If the burning area is not large, then you can cover the burning areawith sand, dirt, or other solid incombustible solid material to separate theburning material from the air.
2. Cooling method: Dump water onthe burning material to lower the temperature to below the combustion point.
3. Removal method: Remove theburning material from the fire source to reduce the burning area.
4. Suppression method: remove thechain reaction. Introduce materials that can interrupt the chain reaction. Thisusually refers to the use of chemical agents, such as dry chemicalextinguishers, on the fire to produce halogen (or alkali metal) ions and effectivelyremoving hydrogen or oxygen from the fire and blocking combustion.







 334007 桃園市八德區介壽路二段901巷49弄35號
334007 桃園市八德區介壽路二段901巷49弄35號
 星期二 - 星期六 |09:00 - 12:00;13:30 - 16:30
(國定連假休館)
星期二 - 星期六 |09:00 - 12:00;13:30 - 16:30
(國定連假休館)
 (03)365-5119
(03)365-5119






